|
GENUV to commence P1/2a Clinical Trial for ALS Drug which Shows Efficacy in Neurogenesis and Neural Homeostasis Recovery
4 hospitals in Korea begin patient recruitment..Comparing safety and efficacy with “Riluzole”
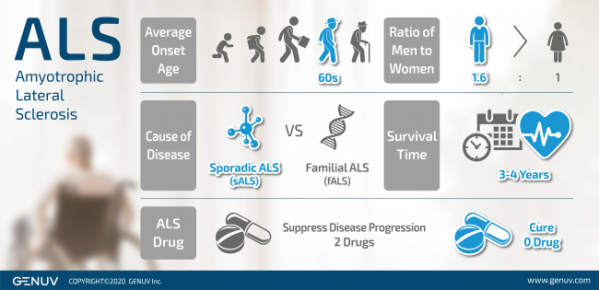
GENUV is to begin a phase 1/2a clinical trial on its Amyotrophic Lateral Sclerosis (ALS) drug candidate, SNR1611, the company’s lead pipeline.
GENUV announced on 13 April 2020 that the company has recently received IRB (Institutional Review Board) approvals from four hospitals in Korea for the clinical trial of an ALS drug candidate, SNR1611, and started patient recruitment.
With the belief that therapeutics generating new neurons (“neurogenesis”) and inducing “neural homeostasis” would be effective in treating neurodegenerative diseases including ALS, GENUV identified and developed SNR1611 through drug repositioning. After initial IND approval from the Ministry of Food and Drug Safety to conduct clinical trials at three sites, GENUV received approval for an additional site during the clinical trial preparation process, resulting in IRB approvals from four major medical centers (i.e., Samsung Medical Center, Yonsei Severance Hospital, Seoul Asan Medical Center and Korea University Anam Hospital).
The phase 1/2a clinical trial will enroll patients showing symptoms started within 2 years of the screening visit and evaluate the safety, tolerance and efficacy of SNR1611 in comparison to the already approved treatment, “Riluzole.” As of 10 April 2020, Samsung Medical Center, Korea University Anam Hospital and Seoul Asan Medical Center initiated the recruitment of patients, and Yonsei Severance Hospital will begin recruitment this month. GENUV, commented, “we hope to find evidence through this clinical trial that SNR1611 has the potential to become a treatment which can provide real help to ALS patients and their family members desperately seeking new treatment.”
....
To view original article, please click here.
|
