GENUV, Research for the Development of “Asymmetric Bispecific Antibody Platform”
Research Collaboration Agreement with Industry University Cooperation Foundation -Hanyang University (Prof. Seong Eon Ryu)
GENUV, a platform technology-based new drug development company, announced on November 1st the execution of the Research Collaboration Agreement with Industry University Cooperation Foundation – Hanyang University for the development of “Asymmetric Bispecific Antibody Platform” technology.
The Agreement provides for GENUV and Prof. Seong Eon Ryu (Hanyang University, College of Life Science) to develop an asymmetric bispecific antibody platform that generates antibodies which can simultaneously bind to two different types of antigens yet have similar properties to natural monoclonal antibodies, leading to superior efficacy and safety.
Prof. Ryu has been studying structural function of disease-related proteins through computer-aided structural modeling and structural identification of proteins at the atomic level. Under the agreement with GENUV, Prof. Ryu will conduct research to increase bispecific antibody performance while minimizing the effect on antibody structure through structural modeling.
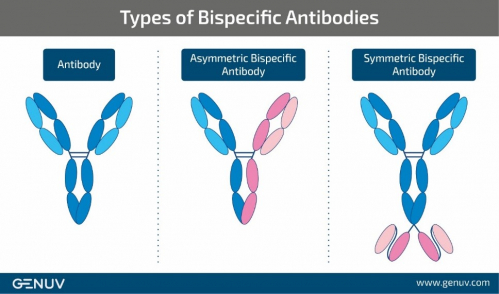
Asymmetric bispecific antibody technology provides a better chance of discovering new drug candidates than symmetric bispecific antibody technology due to its property of allowing a greater variety of combinations; however, R&D proved to be quite challenging due to its high technical difficulties. GENUV, focused on developing original technologies essential for the discovery of new antibody-drugs, recognized the potential use of asymmetric bispecific antibody technology to develop new antibody drugs that could overcome the limitations of existing cancer drug combination therapies and decided to take on the challenge.
...
To view original article, please click here.
|
